
Latest Research Shows How Zinc-Air Batteries Enhance Hydrogen Safety and Efficiency
November 6, 2024 0 By Tami HoodChallenges in Current Green Hydrogen Production
Green hydrogen production is heralded as a cornerstone for a sustainable energy future, yet several obstacles hinder its widespread adoption. The primary challenge lies in the conventional methods of hydrogen production, which predominantly emit carbon dioxide (CO2), negating the environmental benefits of this clean fuel. Additionally, while renewable energy sources like solar and wind power promise a cleaner path, they suffer from inefficiencies due to inconsistent power generation caused by fluctuating weather and temperature conditions.
Another significant challenge involves the need for precious metal catalysts in current systems, which are both economically and environmentally costly. These catalysts degrade rapidly over time, reducing the efficiency and lifespan of hydrogen production systems. Moreover, the risk of fire associated with traditional systems poses safety concerns that must be addressed to ensure practical application.
KAIST’s Innovative Solution to Overcome Challenges
In response to these challenges, researchers at the Korea Advanced Institute of Science and Technology (KAIST) have developed a novel hydrogen production system poised to revolutionize the field. This system, led by Professor Jeung Ku Kang and his team from the Department of Materials Science and Engineering, utilizes a self-powered mechanism based on a high-performance zinc-air battery.
The cornerstone of this innovation is the development of a non-precious metal catalyst known as G-SHELL. This catalyst is synthesized using a nano-sized metal-organic framework grown on graphene oxide, proving effective for multiple catalytic reactions, including oxygen generation, hydrogen generation, and oxygen reduction. By eliminating the dependency on precious metals, the system significantly reduces costs and enhances the longevity of the catalytic materials.
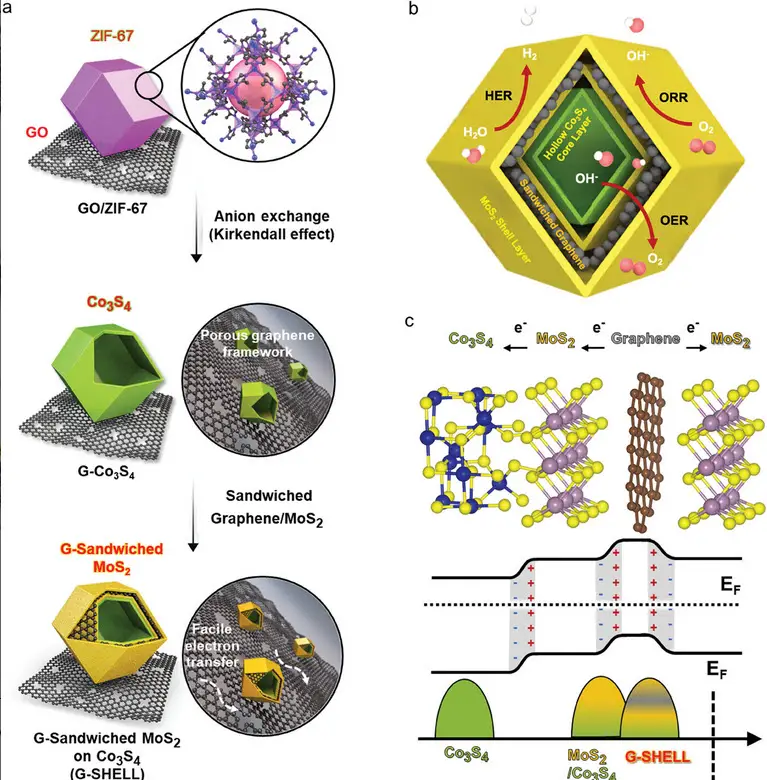
Illustrations of the G-SHELL structure, a trifunctional graphene-sandwiched heterojunction-embedded lattice. This includes: a) the synthesis process from a zeolitic imidazole framework, b) its hollow core-layered shell with trifunctional sites for ORR, OER, and HER, and c) the heterojunctions, induced internal electric fields, and band structure. Image Credit
Furthermore, the zinc-air battery offers a high energy density and exceptional output characteristics, ensuring stable operation even under long-term charging and discharging cycles. The use of a water-soluble electrolyte mitigates the risk of fire, adding a layer of safety to the hydrogen production process.
To Sum it Up This New System Addresses Major Areas Here:
-
High Cost of Precious Metals: Traditional systems rely on expensive metals for catalysts.
- Solution: Utilizes a G-SHELL catalyst made from cost-effective materials, eliminating the need for precious metals.
-
Durability and Performance Deterioration: Catalysts often degrade over time, reducing efficiency.
- Solution: The new catalyst and zinc-air battery are designed for long-term stability, even under repeated use.
-
Low Energy Efficiency: Many systems struggle to efficiently produce hydrogen from water splitting.
- Solution: Employs a high-performance zinc-air battery to enhance energy density and output, improving efficiency.
-
Safety Concerns: Hydrogen production can pose fire risks.
- Solution: Uses a water-soluble electrolyte to mitigate fire hazards, ensuring safer operation.
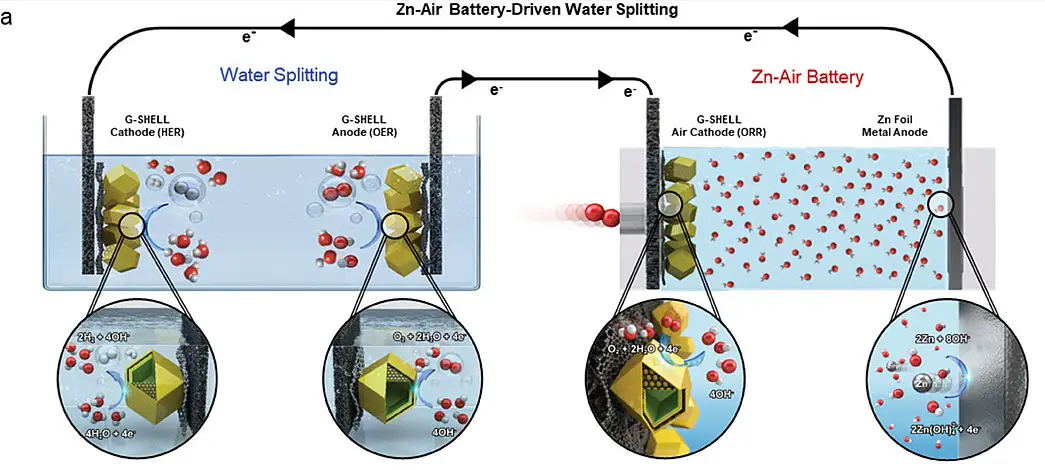
How the New Hydrogen Production System Works
The core of KAIST’s hydrogen production system lies in its sophisticated integration of a zinc-air battery with a water electrolysis setup. The zinc-air battery functions as a robust power source, capable of generating sufficient voltage for efficient water splitting. This process involves breaking down water molecules into hydrogen and oxygen, with the hydrogen collected as a clean fuel source.
The innovative G-SHELL catalyst plays a pivotal role in enhancing the efficiency of this system. Its unique structure, derived from a metal-organic framework on graphene oxide, facilitates the essential catalytic reactions necessary for hydrogen production. This design not only improves the energy density but also boosts the output efficiency, enabling the system to operate effectively even with the variable inputs typical of renewable energy sources.
By addressing the rapid degradation typically seen in traditional catalysts, this system ensures long-term stability and performance. The incorporation of a water-soluble electrolyte further enhances safety by minimizing the risk of combustion, making the system viable for large-scale implementation.



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.